|
Selection and characterization of dermatological preparations
Dermatological preparations are generally selected by dermatologists according to the type of emulsion, lipid content and occlusivity for certain skin conditions. For chronic dermatological disorders, hydrocarbon-based (bases) are often preferred, since especially these preparations have occlusive and protective properties. Although such ointments are extremely useful as emollients, their value as topical release systems (including as magistral formulas) is limited by the poor solubility of many drug substances. Drug substance solubility can be enhanced to a certain extent by formulating them with hydrocarbon miscible solvents like isopropyl myristate or propylene glycol. Anhydrous mixtures are usually very sticky, greasy formulations providing high occlusion on the skin.
 |
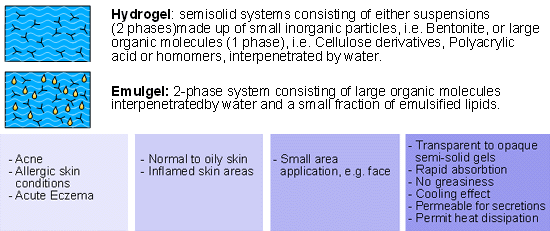
Much more elegant single-phase systems are polar gel formulations. Since these are usually water and / or alcohol based systems (hydrogel, hydro-alcoholic gel) and generally have a low lipid content or are even lipid-free, gels are not favoured for the classical indications of psoriasis and eczema. This type of formulation is appropriate for the application of antiallergics, repellents and antiinflammatories and for the treatment of acne or rosacea.
A wide variety of gelling agents are available for thickening solutions and suspensions, and allow the creation of numerous variations. Besides cellulose derivatives, polysaccharide gums and others, the acrylate polymers (carbomers) are most widely used. The disadvantage of polyacrylate gels is their electrolyte sensitivity, which also compromises their application characteristics on the skin. If a hydrogel additionally contains a dispersed lipid phase, it is called an emulsion gel (cream gel; emulgel), although in pharmaceutical terms it is frequently a quasi-emulsion because the lipid phase is immobilized primarily by the high viscosity of the aqueous phase. The suspension of water-insoluble substances in hydrogels (suspension gel) is another conceivable variant.
 |
Creams (emulsions, aqueous ointments) as disperse systems account for the majority of aqueous formulations. With their readily adjustable properties, they also cover a wide range of applications for numerous dermatological conditions. During emulsion formation, water can appear as both the outer phase (continuous) and as the inner, discontinuous phase. In the first instance they are referred to as oil-in-water (O/W) emulsions, in the second as water-in-oil (W/O) emulsions. The choice of emulsion type is of a certain importance for the treatment or care of the skin in the different indications. Water-in-oil emulsions essentially correspond to the physiological conditions, because the skin's hydrolipid film always takes the form of a W/O emulsion. The W/O emulsion type is the more effective from the dermatological viewpoint because many active ingredients tend to be soluble in lipids and can generally be made more bioavailable. These emulsions also promote the transport of moisture into the epidermis, an effect assisted by the slight occlusion provided by the greasy film after application. Cosmetic acceptance of the W/O films is generally less than for oil-in-water emulsions which allow lipid mixtures to be used in finely dispersed form, which greatly reduces their sometimes sticky and greasy properties. The direct contact between the outer aqueous phase and the skin also increases the evaporation of the water which has a cooling effect after application. On the other hand, it has been found that O/W emulsions frequently withdraw moisture from the skin prematurely because of the surfactant-like properties of the necessary emulsifiers. This is because after the water evaporates, the frequently used PEG emulsifiers dissolve the skin's natural oils. Particular importance therefore attaches to the choice of emulsifiers and the use of moisture binding substances in these cases. O/W creams/lotions nevertheless account for the majority of the products on the cosmetic market, as they are generally preferred by consumers because of their favourable application properties. For use on healthy skin, O/W emulsions, especially when formulated with moisturizers and skin compatible lipids, are basically a good choice in terms of effectiveness for skin care and in the anti-aging segment.
The dispersion of oil droplets in water (O/W) or water droplets in oil (W/O) is more complicated in reality than can be imagined schematically. The arrangement of the numerous emulsifier films which act at the interfaces between the two mixed phases is of central importance for the structures of emulsions. These multimolecular structures form crystalline or liquid-crystalline regions and exhibit hexagonal or lamellar structures and thus influence the phase distribution.
 |
|
 |
Absorption bases are ointment bases which in their system characteristics occupy a position between aqueous and anhydrous systems. These bases are offered by many manufacturers for use in magistral formulations. Most of them are mixtures of hydrocarbons (paraffin, vaseline) used in combination with e.g. PEG emulsifiers, lanolin alcohols, glyceryl fatty acid esters and/or fatty alcohols to achieve higher rates of water absorption. Lanolin alcohols are variable mixtures of liquid and solid fatty alcohols, fatty acids and wax esters of animal origin which have been found to have a sensitizing effect even on healthy skin of humans, especially in children. This allergenic potential is attributable to the lanolin alcohols present in these formulations. Lanolins with a greatly reduced lanolin alcohol content and which have hardly any appreciable sensitizing potential are now available. Since lanolin mixtures exhibit very good skin compatibility as well as good moisturizing properties, they are very useful for dermatological preparations. Unfortunately, it is relatively difficult to gain acceptance for lanolin derivatives because of the negative image associated with their industrial development.
 |
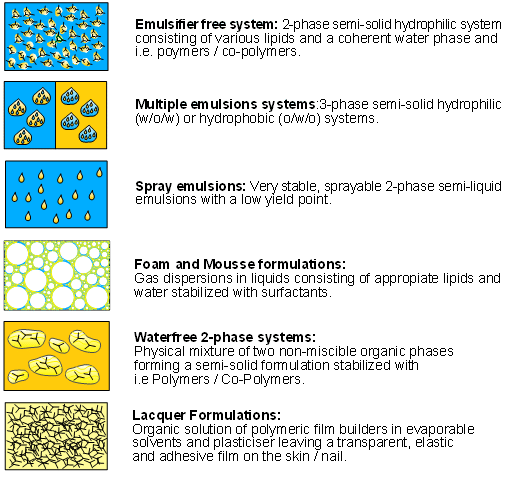
Emulsifier-free systems were developed in the cosmetic industry a considerable time ago. In this context the term "emulsifier-free" indicates the absence of classical emulsifiers/surfactants.
In emulsifier-free systems the lipid phase is always dispersed in water. They are stabilized with, for example, crosslinked acrylic acid derivatives (e.g. acrylates/ C10-30 alkyl acrylates cross-polymers, Pemulen®), whose lipophilic domains are anchored in the oil phase; the more hydrophilic molecular residues of the macromolecules are present in the aqueous phase. As a result, these compounds also exhibit surface activity. After neutralization of their acidic groups, the excipients also have pronounced swellability which, because of the resulting increase in the viscosity of the aqueous phase, gives the formulation additional stability. The particle size of the dispersed inner phase is much greater compared to conventional emulsion systems, although this usually has no impact on the cosmetically attractive product properties of emulsifier-free emulsion systems. Only the electrolyte sensitivity of crosslinked acrylic acid derivatives can adversely impact the application properties, as is the case with the carbopols. After application on the skin, the gel structure formed as a result of swelling is completely destroyed by the ions contained in the sweat of the skin. The free aqueous phase present on the skin after breakdown of the gel structure rapidly evaporates, with the result that when a well-spreading lipid component is chosen a thin film of lipid remains on the skin. Active agents dissolved in this phase can therefore be bound effectively on the upper skin layers.
Another advantage of emulsifier-free systems is their good sprayability. This property in combination with the absence of classical emulsifiers makes it possible to develop cosmetically attractive sunscreen products.
 |
Multiple emulsions are emulsions of the oil-in-water-in-oil (O/W/O) type. Ideally, three-phase systems offer the possibility of separating incompatible ingredients with similar physico-chemical properties by compartmentation.
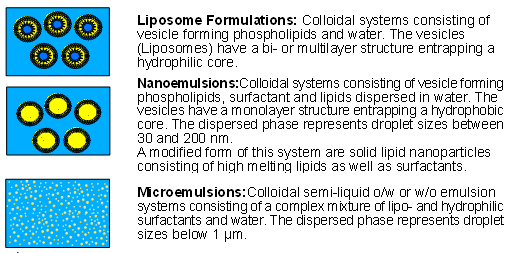
Liposomes are colloidal systems consisting of vesicle-forming phospholipids. The vesicles can be uni- or multilamellar (single or multi-layered), always with an enclosed hydrophilic nucleus.
In contrast, nanoemulsions have a hydrophobic nucleus surrounded by phospholipids and/or surfactants. One special form is solid lipid nanoparticles (SLN), which contain a solid lipid nucleus.
Both liposomes and nanosomes are usually present in an aqueous phase which can be incorporated in semi-solid formulations as required.
These intensively researched systems were originally developed as drug delivery and drug targeting systems. In most cases these high expectations were not fulfilled, however, a situation reflected in the relatively limited number of available formulations based on these systems. Liposomes can be interesting for water-based topical systems in which sparingly water-soluble compounds are to be incorporated in low dosages (e.g. sprays). Despite many formulation attempts and numerous application trials, nanosomes and solid lipid nanoparticles have not yet acquired any importance in the development of semi-solid medicinal products.
The excipients used for liposomes are very cost-intensive, an additional factor which greatly restricts the use of such vehicles. The industrial production of liposomal formulations is further hindered by the expensive manufacturing process and the more demanding stability tests compared to those performed for other semi-solid pharmaceutical preparations.
Microemulsions because of their composition, a complex mixture of emulsifiers and co-emulsifiers, offer good dissolution properties for sparingly soluble active ingredients. In addition, the choice of a suitable emulsifier system can provide an enhancer effect. A definite disadvantage of these formulations is the high skin irritation potential associated with the emulsifier system. The low cosmetic elegance of these formulations is a further drawback. Consequently, microemulsions are of only secondary importance both in dermatology and cosmetics.
Foam formulations offer not only good spreadability but also the advantage of pressure-free application on the skin. This is particularly beneficial for highly damaged areas of skin (e.g. burns). Even formulations of little cosmetic elegance like hydrocarbons can be transformed into a cosmetically elegant form by foaming.
 |
|
